"Any practice that can reduce the number of cesarean deliveries is highly worthwhile."
Dr. Dana Gossett, MD, MSCI
Professor and Chair of Obstetrics and Gynecology, UCSF
Oxytocin administration is widely used to augment labor to achieve a successful vaginal birth, particularly for nulliparous patients who have received epidural anesthesia.
According to ACOG, the side effects of oxytocin are primarily dose related.
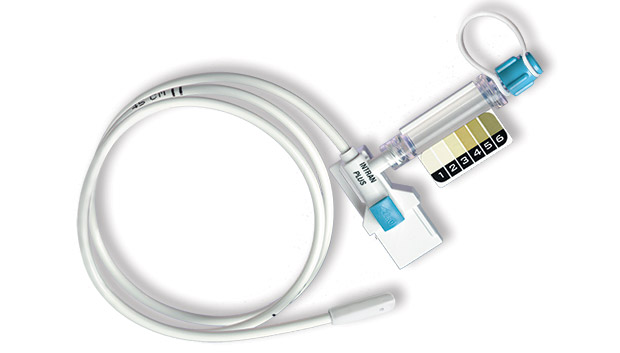
UTMD developed, and in 1991 released for marketing, the seminal disposable IUP monitoring catheter, Intran® Plus. Since 1991, over 5 million Intran® Plus IUPC's have been used without a single reported serious injury.
The primary purpose of Intran® Plus is to provide continuous, accurate measures of contraction intensity, including peak pressure and resting tone, reliably over the course of active labor, that will allow clinicians to effectively titrate the oxytocin dose.
PRODUCT DESCRIPTION
A pre-calibrated, single use transducer in the distal (in-utero) tip of each Intran® Plus catheter converts mechanical pressure at its source in the womb to an electrical signal reliably conveyed to an external monitor. The pressure transducer of Intran® Plus is encapsulated within a soft boot designed for safe placement. Contact with maternal or fetal tissues does not obstruct the intrauterine pressure detection.
The Intran® Plus design incorporates Sensaflex™ tubing that optimizes insertion safety. Sensaflex™ is a tactile tubing material that is stiff enough to allow insertion while maintaining the pliability necessary to avoid unwanted injuries. Combined with the soft, blunt tip of Intran® Plus, Sensaflex™ tubing allows clinicians to sense tissue obstructions prior to causing injury.
The catheter consists of a second lumen which allows simultaneous amnioinfusion while monitoring contractions. "Amnioinfusion is sometimes used to attempt to resolve variable FHR decelerations during the first stage of labor by alleviating umbilical cord compression as a result of oligohydramnios. Amnioinfusion has been found to significantly resolve patterns of variable decelerations..." (KR Simpson and P Creehan in conjunction with AWHONN, Perinatal Nursing, Edition 3). "In addition to decreasing the recurrence of variable decelerations, amnioinfusion has been shown to decrease the rate of cesarean delivery for abnormal FHR patterns." (ACOG Practice Bulletin No 116, November 2010)
All Intran® Plus configurations are designed with a clear covering over the lumen used for amnioinfusion, also called the Amniolumen. Clinicians may use the clear Amniolumen to access amniotic fluid return upon IUPC placement or for visualization of fluid during the course of labor. Some Intran® Plus configurations include the Amnio View Port that provides a convenient means of amniotic fluid visualization. The Amnio View Port includes a meconium color scale to facilitate observation of changes in meconium color over time.
Options for tip size and location of zeroing switch are provided for clinician preference.
FEATURES
INDICATIONS
The Intran® Plus intrauterine pressure monitoring catheter is for use on patients requiring intrapartum pressure monitoring.
CONTRAINDICATIONS
Do not use Intran® Plus if there is uterine bleeding of undetermined origin, or if placenta previa is diagnosed or suspected.
For more information, please contact us by EMAIL or call 1-800-533-4984 | 1-801-566-1200.
© Copyright 2024 Utah Medical Products, Inc. All rights reserved.